Joe Dawson from the University of Essex has penned this guest blog detailing some exciting research that BASS is pleased to support. Details of how BASS members and recreational sea anglers can get involved will be released soon.
A team from the University of Essex have been making their way around the UK coastline this summer sampling juvenile bass, and have already met with interested and engaged bass anglers and members of the public who have been wondering what on earth they are up to!
The juvenile bass are being sampled for two projects funded by the UK government, one from the UKRI Future Leaders Fellowship scheme and the second from DEFRA’s Fisheries Industry Science Partnerships (FISP), awarded in collaboration with the Bass Anglers Sportfishing Society. The projects benefit from broad collaboration with scientists at CEFAS, the Environment Agency, and the University of Southampton, Plymouth and Bangor, as well as several important community and NGO partners. The projects use different methods (which we will detail later in this article!) but share a common goal of understanding the population structure and connectivity patterns of seabass, and the key nursery areas supporting the adult fishery. To reduce unnecessary mortality and to maximize the information we get from every sample, the researchers are joining forces and creating a bass “tissue bank” (sample archive) so that they can use the same fish while also making them freely available for other studies in the future.

Juvenile fish sampling can trigger all sorts of reactions in those watching from a beach or roadside. We did discuss ways we might be able to hide this work away from the public, but due to the need to use specific tides and the many sites being sampled, we are often at the beaches and estuaries used by the public and anglers for their recreation. Children love to see the fishes, and the vast majority of the fish we catch are counted and released to swim away. But not all species fare well on landing (mostly the hyper-abundant ones like sand smelt and sprat) and we do retain some 0-1 year old bass for the tissue bank (around 20 per site), which can lead to different reactions. We understand these reactions and do our best to communicate who we are, how we minimise impact, and why we are conducting our sampling, but we thought this would be a great place to let you all know more details about our work.

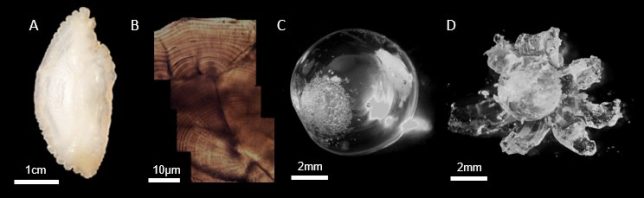
Now we will give you some details about the methods we use. The first project focuses on natural chemical markers from the water and the fish’s food that are recorded in the tissues of the fish as it grows, and can be used to reconstruct its lifetime movements and diet. The tissues we are using for this project are otoliths (calcified ‘ear stones’ that the fish uses for balance and hearing) and eyes lenses (protein spheres inside their eye ball). Both tissues grow continuously over the fish’s life, forming layers just like a tree trunk (see images below), and “documenting” the fish’s environment from the moment they hatch. At the moment we are collecting juvenile reference samples and will analyse their growth layers to build a ‘chemical map’ to identify each estuary. Once these cohorts have reached adulthood, we will sample the adults and analyse their innermost layers to determine which estuary they grew up in. This method gives us the rare opportunity to quantify the relative contributions of different nursery grounds to the adult fishery, and to gather elusive information about their early life histories (e.g. movement and growth), even when they are too small to tag. Overall, we hope that this project will provide important information about which estuaries are most productive (e.g. to earmark for protection measures) and which are performing less well (e.g. to earmark for remediation) to contribute to the long-term conservation goals of the species.
Another exciting aspect of this work involves extracting DNA from the same individuals to assess the genetic structure of juvenile UK bass populations. Emerging genomic technology means it is now possible to discern fine-scale connectivity and population structure in a much more cost-effective manner than previously. This can lead to novel findings which can benefit both stakeholders and fish populations. For example, the identification of genetic barriers (which are not always physical structures like dams, they can also include temperature and contaminants) and isolated populations that are more vulnerable to overfishing, which could result in changes to management methods in order to conserve important genetic variation. Note that a key part of our collaboration with BASS on this project is for the collection of tissues from adult bass. We are currently working with BASS to put out a call to BASS members to help us collect a sample whenever you do keep a fish for the table. This is UK-wide so everyone is welcome to get involved and we will be in touch about this soon!
In summary, while lethally sampling juveniles may seem counterintuitive to protecting this valuable species, sampling low numbers can be one of the safer ways to extract critical data from a population. The average female bass produces 250,000-500,000 eggs per kg of body weight per spawning season, of which only a tiny fraction survive even to age 2. Thus, removing a small number of juveniles at a stage when natural mortality is so high will not have any detectable effects on the stock. That being said, juvenile seabass are rightfully well-protected in this country, and we have received all relevant permissions to undertake this work from MMO and regional IFCA teams.
So please look out for us when you are out fishing, say hello and introduce yourselves. We really do love to meet you and your families and to talk fish, and we sincerely hope the data we collect in this project will lead to you running into more >42cm bass in the future!
Meet the team! Joe Dawson is a Research Assistant and field lead for the two projects, and spent his MSci at Bangor analyzing bass age and growth. Tom Cameron is a Senior lecturer and fish/shellfish expert with a passion for linking science to policy. Anna Sturrock (lead of UKRI project) is a lecturer and Future Leaders Fellow using chemical tracers to understand fish behaviour and health. Michelle Taylor (lead of FISP project) is a Senior Lecturer using genomics to elucidate the population structure of organisms ranging from baby bass to deep sea corals!
